A previously unknown subgroup of uterine fibroid tumours is driven by mutations that result in disruption of the DNA–protein complex chromatin. The findings could inform the management of this common condition
Read the paper: Deficient H2A.Z deposition is associated with genesis of uterine leiomyoma
Previous work has identified at least three mutually exclusive categories of UL, defined according to the genetic alterations they show: those with mutations in the gene MED12 (70%); those in which HMGA2 is activated (15%); and tumours in which FH is mutated (1%)4. However, a subset of ULs do not harbour any of these alterations. To characterize the molecular subgroups of ULs more comprehensively, Berta et al. used various molecular techniques to study the genomes of 2,263 tumours from 728 women.
The authors identified the previously known molecular subgroups in the sampled tumours, and used RNA sequencing to assess gene expression in subgroup-representative tumours and in all available tumours with unknown drivers. Nearly 40% of the tumours from the latter group showed high expression of HMGA1 — perhaps not surprisingly, given earlier work5 implicating HMGA1 alterations in UL. More interestingly, the authors also identified a previously uncharacterized subclass of UL in this ‘unknown driver’ category. Tumours in this subclass carried alterations in the genes encoding proteins that make up the SRCAP complex, which is involved in remodelling of the genetic material in the nucleus.
DNA is packaged up in the nucleus in the form of chromatin. The DNA strand is wrapped around protein cores, each consisting of eight histone subunits, to form structural units of chromatin called nucleosomes. The SRCAP complex is an epigenetic remodeller: it regulates the structure of chromatin without altering the sequence of DNA bases. Specifically, it catalyses the incorporation of the histone variant H2A.Z into chromatin6. H2A.Z is involved in the regulation of gene transcription, the maintenance of genome integrity and DNA repair. Overexpression of H2A.Z is implicated in several types of cancer6.
Berta et al. found alterations in six of the nine genes that encode proteins in the SRCAP complex, with YEATS4 being the most commonly altered gene. Inactivation of both copies of SRCAP-complex genes was a common finding (Fig. 1). This inactivation was caused either by loss of the non-mutated copy of the gene or, in the case of YEATS4 alterations, by epigenetic silencing of the remaining copy of the gene. Moreover, the authors identified six individuals who had at least two tumours with mutations in SRCAP-complex genes, suggesting that certain individuals might be particularly predisposed to such alterations, perhaps because of environmental factors or because of inherited genetic variants, known as germline alterations.
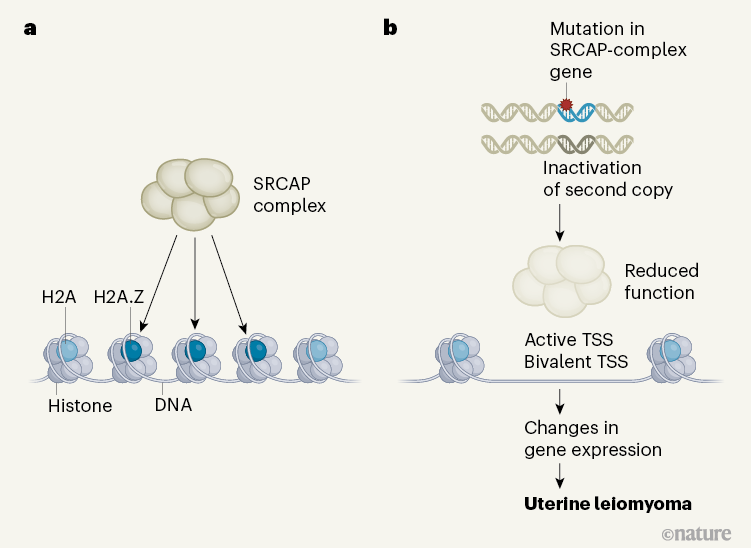 Figure 1 | A molecular mechanism underlying formation of uterine leiomyoma tumours. a, The SRCAP complex of proteins remodels chromatin (DNA wrapped around complexes of histone proteins) by loading it with the histone H2A variant H2A.Z, which regulates gene expression. b, Berta et al.3 examined genomic alterations in 2,263 uterine leiomyoma tumours from 728 women. Some tumours were driven by mutations in genes encoding protein components of the SRCAP complex, combined with another ‘second-hit’ genetic alteration (such as a deletion).
Figure 1 | A molecular mechanism underlying formation of uterine leiomyoma tumours. a, The SRCAP complex of proteins remodels chromatin (DNA wrapped around complexes of histone proteins) by loading it with the histone H2A variant H2A.Z, which regulates gene expression. b, Berta et al.3 examined genomic alterations in 2,263 uterine leiomyoma tumours from 728 women. Some tumours were driven by mutations in genes encoding protein components of the SRCAP complex, combined with another ‘second-hit’ genetic alteration (such as a deletion). The resulting inactivation of both copies of a SRCAP-complex gene can reduce SRCAP-complex function. This leads to deficient loading of H2A.Z at exposed regions of chromatin that contain transcription start sites (TSSs) that are active and bivalent (that is, bearing repressive and activating regulators).
Overall, this results in underexpression of some genes and overexpression of others — including certain genes involved in the spatial organization of growing tissue.
The authors therefore studied germline alterations in the protein-coding portion of the genomes of 25,506 women, stored in the UK Biobank. They found that mutations predicted to reduce the function of the proteins encoded by YEATS4 and another SRCAP-complex gene, ZNHIT1, were strong candidates for an increased risk of UL. The authors validated the UL risk associated with such mutations in a replication group of 78,905 women, obtained from the UK Biobank. Remarkably, in both groups overall, the number of these germline alterations in SRCAP genes was greater than the number of FH mutations, which are well known to predispose women to UL7.
Given the role of the SRCAP complex in loading H2A.Z into chromatin, the authors examined H2A.Z status in samples of myometrium (normal uterine wall) and ULs. SRCAP-altered tumours showed a striking loss of H2A.Z, whereas myometrium, MED12-mutated tumours and FH-mutated tumours showed strong expression, and HMGA1– or HMGA2-altered tumours had moderate expression.
An epigenetic tipping point in cancer comes under the microscope
The authors then analysed H2A.Z binding to chromatin, making use of chromatin immunoprecipitation (ChIP) sequencing, a method that analyses protein–DNA interactions. Compared with myometrium, SRCAP-altered tumours showed reduced H2A.Z–chromatin binding, compatible with the loss of H2A.Z protein expression in this subgroup. Intriguingly, MED12-mutated tumours also had a global decrease in H2A.Z–chromatin binding, despite having strong H2A.Z expression. HMGA2-altered tumours that had lower H2A.Z protein expression than normal also showed decreased binding of H2A.Z.
Findings of a different assay evaluating chromatin organization were complementary to the ChIP-sequencing results. Overall, Berta et al. observed that chromatin activation (whereby the DNA is exposed) in YEATS4-mutated tumours preferentially occurred at transcription start sites (TSSs) that were active or bivalent (that is, bearing both repressive and activating epigenetic regulators). These activated chromatin regions showed reduced H2A.Z binding compared with that in myometrium. These findings are in line with previous animal studies reporting that the loss of SRCAP-complex function results in reduced H2A.Z–chromatin binding6. In association with this epigenetic modification at these TSSs, YEATS4-mutated tumours had changes in the expression of various genes (Fig. 1).
Many of the genes showing increased expression were associated with the spatial organization of cells in growing tissues.
All UL subgroups exhibited increases in the expression of three sets of genes: those encoding the CBX2, CBX4 and CBX8 protein components of the canonical polycomb repressor complex 1 (cPRC1) that epigenetically silences genes to regulate development; genes encoding the developmental transcription factors SATB2 and HOXA13; and genes encoding the enzymes SRD5A2 and HSD17B6, which synthesize the sex hormone dihydrotestosterone. By contrast, expression of the gene encoding CBX7, also a component of cPRC1, was reduced.
Given that mutations in MED12 are the most common genomic alterations in UL, it is interesting that MED12 protein was previously shown to act with CBX7-containing PRC1 to repress the expression of genes involved in mouse cell differentiation8. Berta and colleagues’ findings suggest that, regardless of the mutation status of the tumours, altered PRC1 function might lead to abnormal differentiation of cells that are encouraged to divide by overexpressed developmental transcription factors, potentially leading to UL formation. In addition, inhibiting the enzymes SRD5A2 and HSD17B6 might be a potential therapeutic strategy for treating some ULs.
Mutation alters chromatin changes during injury response to drive cancer
Berta and colleagues’ study is a tour de force in the molecular subclassification of UL. Some questions remain, however. Although there are some hints of the presence of H2A.Z alterations in UL subgroups without SRCAP-complex mutations, the underlying mechanisms are still unknown. Even in SRCAP-altered tumours, a detailed understanding of how ULs might result from diminished H2A.Z–chromatin binding requires further study. The reasons why some individuals accumulate SRCAP-complex-deficient ULs are also not obvious, although the germline-alteration findings suggest a hereditary component. However, environmental factors, such as changes in an individual’s hormonal milieu, might also affect the abnormal differentiation of the bivalent regions of the myometrium genome, and warrant further study. Moreover, SRCAP-complex genes are not currently targeted by clinical sequencing assays, so it might be challenging to translate these findings into routine clinical practice.
Uterine leiomyomas affect millions of women and cost more than US$1 billion in health care annually in the United States2. A comprehensive understanding of the genomic underpinnings of the distinct molecular subgroups of ULs might eventually inform clinical decision-making, from diagnosis to therapy. Berta and co-workers’ study describes how SRCAP-complex alterations lead to decreased loading of H2A.Z into chromatin in UL, recapitulating observations in previous model systems6.
The association of germline SRCAP-complex gene mutations with the predisposition of women to develop ULs not only further supports the authors’ genomic findings in the tumours, but also can have immediate clinical implications for the genetic counselling of affected women and their family members. Through multiple layers of ‘-omics’ data, the authors suggest an epigenetic mechanism for UL development whereby deranged chromatin leads to the expression of genes involved in tumour formation.
doi: https://doi.org/10.1038/d41586-021-02005-8
References
1.Baird, D. D., Dunson, D. B., Hill, M. C., Cousins, D. & Schectman, J. M. Am. J. Obstet. Gynecol. 188, 100–107 (2003).
PubMed Article Google Scholar
2.Morgan, D. M. et al. Am. J. Obstet. Gynecol. 218, 425–425 (2018).
PubMed Google Scholar
3.Berta, D. G. et al. Nature https://doi.org/10.1038/s41586-021-03747-1 (2021).
Article Google Scholar
4.Mehine, M., Mäkinen, N., Heinonen, H.-R., Aaltonen, L. A. & Vahteristo, P. Fertil. Steril. 102, 621–629 (2014).
PubMed Article Google Scholar
5.Williams, A. J., Powell, W. L., Collins, T. & Morton, C. C. Am. J. Pathol. 150, 911–918 (1997).
PubMed Google Scholar
6.Giaimo, B. D., Ferrante, F., Herchenröther, A., Hake, S. B. & Borggrefe, T. Epigenet. Chromatin 12, 37 (2019).
PubMed Article Google Scholar
7.Tomlinson, I. P. M. et al. Nature Genet. 30, 406–410 (2002).
PubMed Article Google Scholar
8.Papadopoulou, T., Kaymak, A., Sayols, S. & Richly, H. Cell Cycle 15, 1479–1493 (2016).
PubMed Article Google Scholar